A collaborative study between the Medical Sciences Division and the Mathematical, Physical and Life Sciences Division at the University of Oxford has been awarded the 2024 PNAS Cozzarelli Prize in Biomedical Science.
The research, ‘Ultrahigh frequencies of peripherally matured LGI1 & CASPR2-reactive B cells characterise encephalitis patient cerebrospinal fluid’, published in Proceedings of the National Academy of Sciences PNAS, sheds new light on the molecular mechanisms behind autoimmune encephalitis (AE) – a rare but serious neurological condition.
Led by Professor Sarosh Irani (Nuffield Department of Clinical Neurosciences, now employed at the Mayo Clinic, Florida), the study brought together researchers across disciplines to investigate the properties of antibodies that cause AE. The team analysed over 150 antibodies isolated from the cerebrospinal fluid (CSF) of affected patients and discovered that, remarkably, around 80% were reactive to just two self-proteins: LGI1 and CASPR2.
‘I am very excited to see that my team won this prize for our publication, as it captures the essence of our patient-to-bench translational research programme,’ said Professor Irani. ‘Through our research, we found cells – which likely directly contribute to the patient’s autoimmune disease – at ultrahigh frequencies in the CSF. These form a potential target for improved therapeutics going forward.’
Researchers from the Oxford Protein Informatics Group (Department of Statistics), Dr Matthew Raybould and Professor Charlotte Deane, supported the bioinformatics analysis that characterised the antibodies’ genetic diversity and developmental origins. Their work helped reveal that the B cells likely began gaining self-reactivity while circulating in the bloodstream, before migrating to the central nervous system where they transformed into long-lived antibody-producing cells.
Dr Raybould said: ‘It was fascinating to learn about the pathways driving AE. We are humbled to receive this recognition and excited for the future of immunoinformatics. Further development and application of this toolkit to antibody and T-cell receptor data promises to unlock how a spectrum of autoimmune diseases might be intercepted or prevented in the future.’
Professor Deane added: ‘It is an honour to have been part of the team that won this prize. The work is a real demonstration of cross-disciplinary/cross-divisional collaboration and the growing power of computational methods in solving real-world problems.
‘This research showcases how computational methods can help us understand complex biological questions – and ultimately, improve clinical outcomes.’
The Cozzarelli Prize in Biomedical Science is awarded annually to a PNAS publication judged by the academy’s Editorial Board to 'reflect scientific excellence and originality'.
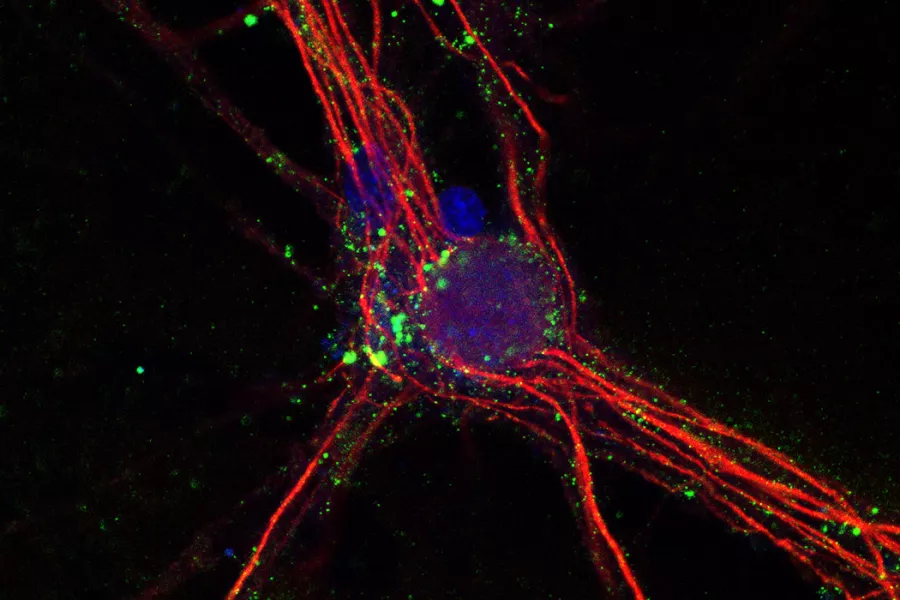
IMAGE: Binding of live hippocampal neurons by monoclonal antibodies created from a cerebrospinal fluid plasmablast extracted from an encephalitis patient. Image credit: Jakob Theorell, Ruby Harrison et al.